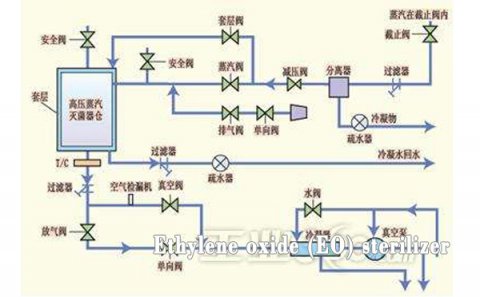
Sterilize machine
This cabinet is generally used for hospital disinfection. The ethylene oxide sterilizer is a special equipment for fumigating and sterilizing the articles closed in the sterilization room under certain temperature, pressure and humidity conditions, using ethylene oxide mixed gas. Compared with the traditional high-pressure steam sterilization, ethylene oxide gas sterilization has the characteristics of strong penetration, broad-spectrum sterilization, thorough sterilization, no corrosion and no damage to articles, and can also be sterilized at lower temperature, pressure and humidity.
Sterilize machine description
|
NO. |
Item name |
Types |
Producer |
|
1 |
IPC |
ADV 610L+ACER 21.5' |
Yanhua automation |
|
2 |
Display |
21〞 |
AOC |
|
3 |
Controller: PLC |
CPU SR60 AC/DC/RLY |
SIEMENS |
|
4 |
Controller: PLC |
EM AE04 |
SIEMENS |
|
5 |
Controller: PLC |
EM AQ02 |
SIEMENS |
|
6 |
centralized control network module |
CSM1277 |
SIEMENS |
|
7 |
LCD(willan) |
MT6071IP |
WEINVIEW |
|
8 |
PPI communication cable |
USB-PPI |
SIEMENS |
|
9 |
General connector |
90 - degree qualification |
SIEMENS |
|
10 |
Field control,data acquisition module,Conductive detection with 4 channels,Digital quantity input at 8 o 'clock,4 switch output, RS485 communication |
CPU SR60 AC/DC/RLY |
SIEMENS |
|
11 |
The door switch in position limit switch |
LJ8A3-8-Z/BY ,2 ones per door |
HUGONG |
|
12 |
Magnetic level gauge turning |
DSUHC-1225 |
HUAIANDESEN |
|
13 |
electronic scale |
XK3150 |
NINGBO LANGKE |
|
14 |
switching power supply |
DRP-120-24 |
TAIWAN MINGDE |
|
15 |
low-voltage apparatus |
Transformer filter circuit breaker contactor relay heat the buttons and indicator lights etc. |
SHINAIDE |
|
16 |
Digital electric meter leakage transformer |
PD194E-9S4 |
WENZHOU JIECHENG |
|
17 |
Install attachment |
In terminal guide trough cabinet control wire and power wire |
HDX |
|
18 |
Control cabinet |
Key type |
HDX |
|
19 |
Printer |
laser printing type 1020 |
HP |
Overview
Disposable sterile medical devices are directly in contact with human tissues and blood, and their safety and effectiveness are directly related to patients' life and health and safety. A kind of ethylene oxide sterilization is a special process to ensure product quality. Sterilization confirmation is the premise and important link to ensure the effectiveness of product sterilization.
To standardize the confirmation and routine control of ethylene oxide sterilization is not only the need of enterprises to ensure the quality of disposable sterile medical devices, but also the need of implementing national medical devices and quality certification of medical devices.
Concept of sterilization
The process of making a product free of any type of viable microorganism. That is to use physical and chemical methods to kill all microorganisms on the transmission medium and make it sterile.
Characteristics of ethylene oxide
Ethylene oxide (EO), also known as ethylene oxide, has a small molecule, unstable structure of the ternary ring, has a strong penetration and chemical activity. Ethylene oxide is a colorless and transparent liquid at 4 ℃, with a density of 0.884g/ml and a boiling point of 10.8 ℃.
It has an aromatic ether flavor. Ethylene oxide is soluble in any proportion of water, or in organic solvents or oils.
Ethylene oxide is widely used in the sterilization and disinfection process of products because of its high vapor pressure, strong penetration to packaging and sterilization objects, and strong oxidation performance. A kind of
Ethylene oxide is inflammable and explosive. When the content of air is 3% - 80%, it will form explosive gas mixture, which will burn or explode in case of open fire.
The chemical property of ethylene oxide is active, and contact with catalyst can cause chemical reaction, which is accelerated with the increase of temperature, pressure and water quantity, producing yellow viscous substance, easily blocking the pipeline and affecting the sterilization effect.
Ethylene oxide can be mixed with a certain proportion of gases with stable chemical properties (such as CO2 and N2), which can reduce cost and increase safety.
The toxic effect of ethylene oxide on human body is mainly direct contact or inhalation. Ethylene oxide gas can stimulate respiratory tract. In the process of sterilization, protective measures should be taken. If the liquid of ethylene oxide accidentally splashes on the skin or eyes, it should be washed with water immediately.
Mar 08, 2020
view: 1190
Sterilization principle Ethylene oxide (EO) is a highly effective gas sterilizing agent, which has been widely used in the sterilization of heat and humidity sensitive medical devices since 1950s. Its liquid state and gas state have sterili...
Read More
Jul 12, 2023
view: 1012
Epoxy ethane is flammable and explosive gas, especially warned! Ethylene oxide is a kind of flammable and explosive toxic hazardous chemicals, the concentration of more than 3% in the air encounter fire is burning and explosive . Therefore the use an...
Read More