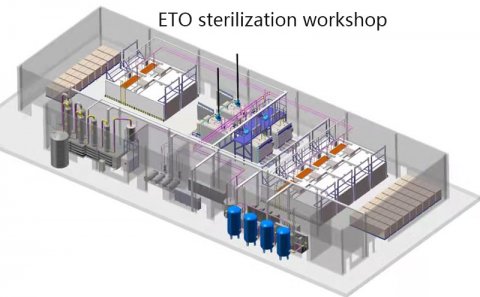
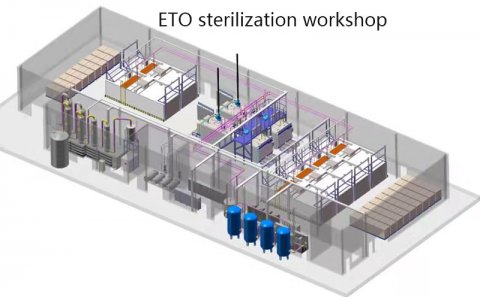
Ethylene oxide sterilizer is a low-temperature sterilization equipment that uses ethylene oxide gas as the sterilization medium, designed specifically for medical devices, electronic instruments, and other items that are not resistant to high temperature and humidity. The core principle is to destroy microbial proteins and nucleic acid molecules through alkylation reactions, achieving broad-spectrum sterilization (including bacterial spores, viruses, etc.) while maintaining the integrity of the material.
The five key elements of an ethylene oxide sterilizer are key process parameters that ensure sterilization effectiveness, and their interactions directly affect the efficiency and safety of microbial killing.
The five key elements are as follows:
1. Temperature
Range: 37 ℃~60 ℃, commonly used optimal temperature is 50 ℃~55 ℃.
Function: Increasing temperature can accelerate microbial metabolic activity, enhance the penetration and reaction rate of ethylene oxide (EO); But exceeding 60 ℃ can easily lead to EO polymerization failure.
2. Humidity (relative humidity)
Range: 30%~80% RH.
Function: Microbial moisture content affects the efficiency of alkylation reaction. Low humidity hinders EO from penetrating the spore coat, while high humidity dilutes gas concentration or causes EO hydrolysis.
3. Ethylene oxide concentration
Range: 300~1000mg/L, commonly used industrial 600mg/L ± 30mg/L.
Function: There is a positive correlation between concentration and sterilization rate (e.g. within the range of 50-500mg/L, the higher the concentration, the faster the microbial death). Insufficient concentration leads to sterilization failure, while excessive concentration increases the risk of residual toxicity.
4. Sterilization time
Requirement: At least twice the microbial killing half cycle, complete spore killing takes ≥ 7.8 minutes (D value needs to reach 2.6-5.8 minutes).
Function: When the time is insufficient, the probability of microbial resurrection increases. If it is too long, it affects the stability of the instrument material and prolongs the analysis cycle.
In addition, with a temperature increase of 10 ℃, the concentration requirement can be reduced by about 30%, and the sterilization time can be shortened by 50%
5. Pressure (vacuum degree)
Function: The pre vacuum depth determines the amount of residual air and affects the ability of EO gas, heat, and moisture to penetrate into the interior of the instrument.
Control points: Negative pressure environment (such as below -50kPa) can enhance gas diffusion efficiency, but it is necessary to avoid negative pressure damage to equipment or packaging
Summary of Operation Points
The five elements need to be dynamically balanced: high temperature needs to be matched with low concentration, high humidity needs to be controlled for a short period of time, and vacuum depth needs to be flexibly adjusted according to the structure of the instrument. Before sterilization, the effectiveness of the parameter combination needs to be verified through a biological indicator (Bacillus subtilis spore) to ensure that the logarithmic decrease in microbial killing is ≥ 6.
Supplementary secondary influencing factors
Loading method: Items should be stacked with gaps (not touching the cabinet wall), and the loading capacity should be ≤ 80% to ensure gas circulation.
Packaging and Material: The thickness of the packaging, the breathability of the material (such as Tyvek), and the structure of the device (such as the complexity of the internal cavity) affect the penetration efficiency of EO.
Microbial load: The higher the initial amount of contaminating bacteria, the longer the required sterilization time
Applicable objects
Medical devices: rigid/flexible endoscopes, implants, catheters, precision surgical instruments.
Special materials: plastic, rubber, silicone products, and optical lenses (non corrosive).
Other fields: cultural relics and ancient books, protective clothing, seed sterilization (strict residue control is required).
Disable Scene
Food, liquids, oils and fats (EO can be residual and toxic).
Powder materials (uneven penetration, potential explosion risk).
Unable to penetrate ceramic and metal products.
In short, ethylene oxide sterilizers have become the core equipment for medical device sterilization due to their low-temperature broad-spectrum sterilization and strong penetration power. When selecting, it is necessary to comprehensively consider the volume, safety configuration, and verification standards (such as compliance with biological indicators), and strictly follow the operating procedures to avoid toxicity risks. Medical institutions recommend medium-sized equipment (such as HMQ-1, HMQ-3), and industrial scenarios can choose large-scale customized systems (such as HMQ-10, HMQ-30, HMQ-50, HMQ-100).
Jun 17, 2025
view: 1773
Storage of ethylene oxide 1 ethylene oxide shall be stored in a separate room. The room shall be equipped with ventilation, explosion-proof and fire-fighting facilities. 2 the ethylene oxide cylinder shall be stored in a cool place away fro...
Read More
Jun 17, 2025
view: 1855
eo sterilizer equipment:Product description. 8-12 hours per sterilization cycle. 2 cycles per day, 0.6-0.8kg ethylene oxide per cubic meter sterilizer. Process: packaging sterilization aeration testing delivery. 1. Layout. need to get the floor plan...
Read More