This article explains the implementation process of the ethylene oxide sterilizer machine project and the detailed steps to determine the sterilizer configuration, it can quickly solve related problems of the EO sterilizer machine project.
Firstly, Understand the process characteristics of ethylene oxide sterilization.
The ETO sterilizaton process is used to sterilize medical devices and other items sensitive to heat or moisture.
The basic ETO sterilization cycle consists of 5 stages (i.e., preconditioning and humidification, gas introduction, exposure, evacuation, and air washes) and takes approximately 8-10 hrs excluding aeration time.
In addition, Determine whether to use ethylene oxide sterilization based on product material and packaging type.
Ethylene oxide gas cannot penetrate ceramics and metals.
For products that are not compatible with high temperatures and ionizing radiation, ethylene oxide sterilization is a good choice. Due to its low-temperature process that can prevent material deformation or oxidation, ethylene oxide sterilization has a wide range of applications in medical device processing.
EO sterilization has the advantage of being compatible with multiple materials and is suitable for products that cannot withstand high temperature sterilization. Ethylene oxide can penetrate into the smallest gaps of the items to be disinfected. This feature allows it to be used on already packaged materials.
Products and packaging made from different materials require different sterilization processes.
Is the packaging made of PE or medical paper? Whether the packaging is vacuum packaging? Vacuum packing is easy to expand or burst when vacuuming during sterilization. Whether there is an aeration/dialysis area on the package?
We will provide the best sterilization process proposal according to the products and packaging.
Based on the type and characteristics of the product, preliminary testing is conducted to determine the duration, temperature, gas concentration, and humidity level of sterilization, in order to achieve effective results without damaging.
More importantly, Once it is confirmed that the product can be sterilized with ethylene oxide, the steps for the ETO sterilizer project are as follows:
Step 1. Calculate the daily production volume of the product to determine the capacity of the sterilizer
The actual capacity of the ethylene oxide sterilizer is about 60%. Normally, a 10 cubic meter sterilizer can hold 6 cubic meters of product. That is to say, if there are 6 cubic meters of items to be sterilized per day, a 10 cubic meter sterilizer can usually be used.
-
Sterilization takes about 8~10 hours.
-
The sterilizer can complete two sterilization cycles per day.
-
The duration of sterilization depends on PQ.
At the same time, the size of the pallets should be determined. The commonly used pallet sizes are: 800mm * 1200mm, 1000mm * 1200mm, 1100mm * 1100mm.
The size of the pallet can be calculated and determined based on the dimensions of the product packaging.
|
Volume |
(L*W*H) |
Sliding door(L*W*H) |
|
Internal Size |
Outer size |
Shipment size |
|
1m3 |
1400*700*1000mm |
N/A |
N/A |
|
2m3 |
1650*1000*1200mm |
2070*2520*1720mm |
2070*1360*1720mm |
|
3m3 |
1400*1350*1700mm |
1820*3220*2270mm |
1820*1710*2270mm |
|
4.5m3 |
2000*1350*1700mm |
2420*3220*2270mm |
2420*1710*2270mm |
|
5m3 |
2250*1350*1700mm |
2670*3220*2270mm |
2670*1710*2270mm |
|
6m3 |
2700*1350*1700mm |
3120*3220*2270mm |
3120*1710*2270mm |
|
8m3 |
3500*1350*1700mm |
3920*3220*2270mm |
3920*1710*2270mm |
|
10m3 |
4500*1350*1700mm |
4920*3220*2270mm |
4920*1710*2270mm |
|
15m3 |
5500*1350*2000mm |
5920*3220*2570mm |
5920*1710*2570mm |
|
20m3 |
7500*1350*2000mm |
7920*3220*2570mm |
7920*1710*2570mm |
|
26m3 |
9600*1350*2000mm |
10020*3220*2570mm |
10020*1710*2570mm |
|
30m3-1 |
11300*1350*2000mm |
11720*3220*2570mm |
11720*1710*2570mm |
|
30m3-2 |
5600*2700*2000mm |
6060*6260*2580mm |
6060*3280*2580mm |
|
35m3-1 |
13000*1350*2000mm |
13420*3220*2570mm |
13420*1710*2570mm |
|
35m3-2 |
6550*2700*2000mm |
7010*6260*2580mm |
7010*3280*2580mm |
|
48m3-1 |
12850*1400*2700mm |
13270*3320*3270mm |
13270*1760*3270mm |
|
48m3-2 |
6550*2700*2700mm |
7010*6260*3280mm |
7010*3280*3280mm |
|
52m3 |
13900*1400*2700mm |
14320*3320*3270mm |
14320*1760*3270mm |
|
55m3 |
7500*2700*2700mm |
7960*6260*3280mm |
7960*3280*3280mm |
|
63m3 |
8650*2700*2700mm |
9110*6260*3280mm |
9110*3280*3280mm |
|
70m3 |
9700*2700*2700mm |
10160*6260*3280mm |
10160*3280*3280mm |
|
78m3 |
10750*2700*2700mm |
11210*6260*3280mm |
11210*3280*3280mm |
|
86m3 |
11800*2700*2700mm |
12260*6260*3280mm |
12260*3280*3280mm |
|
93m3 |
12850*2700*2700mm |
13310*6260*3280mm |
13310*3280*3280mm |
|
101m3 |
13900*2700*2700mm |
14360*3280*3280mm |
12260*3280*3280mm |
Once you have determined the pallet size and EO sterilizer capacity, you can proceed to the next step.
Step 2. Determine the function and configuration of the EtO sterilizer.
After determining the size of the sterilizer, the following items of sterilizer should also be determined:
1. The voltage and frequency of your local industrial power supply.
The power required for sterilizers is usually 380V50Hz. If your local power supply voltage and frequency are not 380V50Hz (such as 415V50Hz, 380V60Hz, 440V60Hz), you can request the use of a transformer or customized electrical equipment.
2. The percentage of ethylene oxide gas.
Determine the concentration percentage of ethylene oxide gas.
The commonly used EO concentration percentages are: 100% EO,90%EO+10%CO2, 80%EO+20%CO2, 70%EO+30%CO2, 30%EO+70%CO2, 20%EO+80CO2, 10%EO+90%CO2, etc.
The lower the EO percentage concentration, the higher the required pressure, the higher the cost of the sterilizer, and the relatively shorter the required aeration time.
EO gas with a concentration of over 80% usually requires nitrogen gas for safety protection, and then it needs nitrogen generator.
-
The EO sterilizers with concentrations ranging from 70% EO to 30% EO are standard configurations.
-
The cost of an EO sterilizer with 20% EO is 10% higher than that of sterilizers with 30% EO.
-
The cost of an EO sterilizer with 10% EO is 30% higher than that of sterilizers with 30% EO.
In some countries, it is not easy to purchase ethylene oxide gas. You can consult the local EO supplier to determine the percentage of EO concentration that can be purchased.
3. Humidity range.
Default humidity: 30%RH-80%RH. Determine whether to customize the humidity range.
Usually, absorbable and non absorbable sutures require a dry vacuum pump and nitrogen generator to ensure that the humidity is not too high.
4. Temperature range.
Default temperature range: 30 ℃ -55 ℃. Limit < 58 ℃. Determine whether to customize the temperature range.
5. Types and quantities of EtO sterilizer doors.
Types of doors: sliding doors, revolving doors, and lifting doors.
The sliding door has the lowest cost and occupies the largest area of the sterilization workshop.
The cost of revolving doors is relatively high. Many customers prefer revolving doors.
The lifting doors save space, but have the highest cost. If the sterilization workshop has a tight area and a high internal height, a lifting door can be used.
Then determine the number of doors, whether it is one or two.
6. Material of sterilizer water jacket.
Material: Carbon steel, 201 stainless steel, 304 stainless steel.
The price of 201SS is about 1.5 times that of carbon steel.
Carbon steel water jacket Service life: more than 20 years.
201SS jacket Service life: more than 30 years.
304SS is suitable for sterilizers with higher requirements, and its price is twice that of carbon steel.
Generally, It is not recommended to use 304 stainless steel as the water jacket for sterilizers with a capacity exceeding 50 cubic meters.
7. Parameter release
Some customers use BI, while others require parameter release.
If parameter release is required, additional SEC sensor and humidity sensor isolation device needs to be installed inside the sterilizer, additional about $15,000.
8. The automatic conveyor device inside the sterilizer.
Some countries require worker safety regulations to prohibit workers from entering sterilizers. In this case, a automatic conveyor of sterilizer can be used.
The internal automatic conveyor of sterilizers can be done for safety and to save manpower.
9. Loading and unloading methods of sterilizers.
The commonly used loading and unloading methods include manual carts and electric forklifts.
You can choose your preferred loading method based on your budget and needs.
10. Special or customized items
To meet the 21 CFR Part 11 compliance standards, it is recommended to add the following configurations:
-
Auxiliary industrial computer ( Auxiliary IPC)
-
Online EO sensor
-
Additionally, it is suggested to add EO monitor of the workshop environment. Sterilization workshop can set up 8 monitoring sampling points to improve workshop safety.
-
Or other items that customers want to customize.
After determining the functional configuration of the sterilizer, the third step can be carried out as follows:
Sep 3. Precondition room.
Regarding the Precondition room, determine if a separate one is needed.
Precondition room can shorten the entire sterilization cycle.
The function of the Precondition room is to humidify and heat the products to be sterilized, This way, the product can be quickly sterilized in the sterilizer, thereby shortening the sterilizer cycle.
If no precondition room, the product will undergo heating and humidification processes in the sterilizer.
If you require a shorter sterilization cycle, you can choose a precondition room.
Step 4. Aeration room.
Regarding the aeration chamber, determine if a separate one is needed.
Degassing/Aeration room can quickly remove some EO residues from the product.
There are usually three ways of aeration:
-
Option A:Aeration in the separate Aeration room for 1-2 days. Generally, Syringes, long catheters, gloves require separate Degassing/Aeration room.
-
Option B:Aeration in the same sterilization chamber. At this point, due to the products to be sterilized being in the sterilizer for 24 hours or longer, it is necessary to expand the capacity of the sterilizer to load more products.
-
Option C:If option A or B is not selected, sterilized products can be placed in a well ventilated warehouse and naturally aerated for 14-20 days. In this case, there is a risk of EO residue not meeting the standard.
Additionally, what is the requirement for EO residue of sterilized products in ppm? 10ppm? or other?
Generally, standard requires the EO residue of products after EO sterilization shall not exceed 10ppm (10μg/g) when leaving the factory.
Finally, the duration of Degassing/Aeration and EO residue depends on PQ.
It is usually recommended to use option A or B to allow for the removal of gaseous residues down to a legally acceptable level.
As mentioned in steps 3 and 4 above, the three stages of heating, sterilization, and aeration can be carried out in the same sterilizer chamber or in three separate chambers/rooms.
Step 5. Nitrogen generator.
Regarding the Nitrogen generator, determine if it is needed.
The function of nitrogen is to ensure dryness and protect safety.
Items sensitive to humidity require nitrogen to ensure dryness.
When the percentage of EO gas exceeds 80%, nitrogen is required to improve safety. Nitrogen can improve safety at all times.
Step 6. EO waste gas treatment equipment/Apparatus.
Catalytic abatement where the equalized concentration of EtO is converted (catalytically oxidized) into CO2 and H2O. At some stage of the process, combustion method was used. Advantage: Non polluting emissions; Disadvantages: High cost and easy to cause fires.
EtO scrubber for EO sterilizer.
Hydrolysis method: Ethylene oxide has a good affinity for water and can form ethylene glycol with water. Ethylene oxide is more prone to form ethylene glycol with water under acidic conditions. We add sulfuric acid to catalyze the production of ethylene glycol during the hydrolysis process of ethylene oxide waste gas, and then neutralize it with alkali, with a pH value close to 7.
Ethylene oxide can hydrate with water:C2H4O + H2O — CH2OH-CH2OH.
Emission standard of Eto Scrubber: 80mg/Nm³ (44ppm), 40mg/Nm³ (22ppm), 5mg/Nm³ (3ppm), 1mg/Nm³ (½ppm).
Material of Ethylene Oxide Scrubber: PPH, FRP,304SS.
Generally, small sterilizers less than 5m³ do not need EO tail gas treatment equipment.
Advantages: Multiple emission standards to choose from, low cost. Disadvantage: Regular disposal of waste liquid is required.
Usually, it is recommended to use EtO Scrubber.
Please consult the local regulatory agency for emission standards.
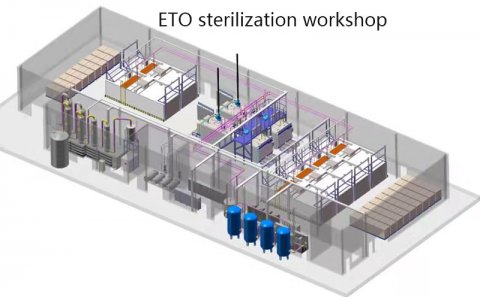
Step 7. Layout and preparation of workshop conditions for sterilization.
Generally, EO sterilizer needs EO Room, Auxiliary Room and Control Room.
Standard requires that the door of EO room cannot lead to sterilization workshop.
-
1. The whole sterilization workshop it is forbidden to open fire, away from the fire. Solid ground level.
-
2. Sterilization workshop shall ensure the temperature over 10 degrees Celsius (heating in winter).
-
3. The sterilizer room should guarantee the well ventilated, use explosion-proof lamps and lanterns and switch.
-
4. The sterilizer room, explosion-proof axial flow fan, installed in 300-500 mm off the ground.
-
5. EO room should be well ventilated, avoid direct sunlight, use explosion-proof lamps and lanterns and switch
-
6. EO room /control room /Auxiliary room height 3000mm(min).
We(Bocon) will quickly provide free sterilization workshop layout design based on customer needs.
From the analysis made above, we can understand the configuration of sterilizer machines and quickly carry out the progress of sterilizer projects step by step.
By the way,we(Bocon) provide a turnkey project plan for sterilizer equipment. Our customers cover more than 30 countries and regions, such as Türkiye, Saudi Arabia, America, Mexico, Brazil, Argentina, Malaysia, South Korea, Indonesia, Vietnam, India, Cambodia, Pakistan, South Africa, Egypt, Nigeria, Algeria, ARMENIA, etc
Finally, any questions regarding the sterilizer equipment project, customers are welcome to consult and negotiate with us.
Jun 17, 2021
view: 1988
The factors that affect the sterilization effect are temperature, humidity, concentration, time, pressure, packaging method, etc. 1. Temperature: The conventional limit of temperature is generally 44 ℃ ~ 56 ℃, and the most commonly used temperatu...
Read More
May 18, 2022
view: 1830
EO gas and its mixture is an effective disinfectant mainly used for sterilization of medical devices which are sensitive to damp heat but can not be sterilized with damp heat. EO is toxic, flammable and explosive. It should be noted that. Ventilation...
Read More