Inspection of mask after sterilization with EO
Any product must pass the sterilization verification procedure to determine the sterilization process!
Sterilization verification is generally carried out by half cycle method. This method is to determine the shortest EO action time without viable bacteria when all other process parameters except time remain unchanged. Two more tests should be repeated to confirm the minimum sterilization time, and both tests should indicate the sterile growth on the biological indicator. The specified action time shall be at least 2 times of the shortest sterilization time. In order to prove the reliability of the resuscitation technology, a short cycle operation should be carried out.
Sterilization time of mask
The conditions used to resuscitate the bioindicators in the validation study should be determined and documented. In determining the culture time, the possibility of delayed growth of spores after EO sterilization should be considered.
Determine parameters:
Temperature 50 ± 3 ℃
Humidity RH 30-80%
EO concentration (pure) 600-800mg / L
Time t (conduct verification and confirmation)
Denifine of oxirane disinfection
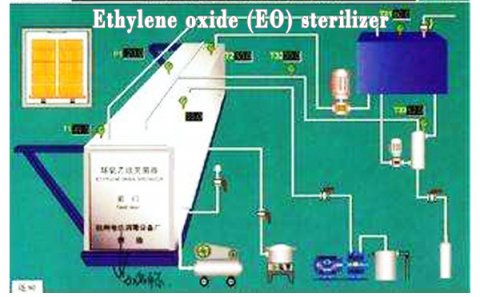
Ethylene oxide sterilization device is the key equipment of disposable sterile medical equipment manufacturing enterprises. There are special requirements for installation, operation and use management. Ethylene oxide is a broad-spectrum sterilization agent, which can kill all kinds of microorganisms, including spores, tuberculosis, bacteria, es, fungi, etc. at room temperature.
Professional and meticulous customer service
The value of an enterprise lies in the happiness of its employees and the moving of its customers. Create a happy material and spiritual home for employees as much as possible. Only a happy team can win satisfied customers. Our team is consistent, constantly challenging ourselves and striving to create value for our customers. Customer satisfaction is the only standard to measure the service quality of our company, because all our employees know that customers' products can be trusted by us. We have the responsibility and obligation to treat customers' products as our own. Whether we control the quality of ethylene oxide sterilization from a professional and perfect management system, or take the handling of goods carefully, we should reassure customers and then deliver them to their customers with satisfaction. This is a win-win situation for all !
The process characteristics of ethylene oxide sterilization.
The ETO sterilization process is used to sterilize medical devices and other items that are sensitive to heat or moisture.
The basic ETO sterilization cycle includes 5 stages (pre-treatment and humidification, gas introduction, exposure, evacuation, and air washing), which require approximately 8-10 hours in addition to aeration time.
In addition, determine whether to use ethylene oxide sterilization based on product materials and packaging types.
Ethylene oxide gas cannot penetrate ceramics and metals.
For products that are incompatible with high temperatures and ionizing radiation, ethylene oxide sterilization is a good choice. Due to its low-temperature process that can prevent material deformation or oxidation, ethylene oxide sterilization has a wide range of applications in medical device processing.
The advantage of ethylene oxide sterilization is its compatibility with multiple materials and suitability for products that cannot withstand high temperature sterilization. Ethylene oxide can penetrate into the smallest gap of the item to be disinfected. This feature allows it to be used for packaged materials.
Products and packaging made from different materials require different sterilization processes.
Mar 06, 2020
view: 1106
Five factors affecting the sterilization of ethylene oxide: temperature, pressure, humidity, ethylene oxide concentration and sterilization time 1、 Temperature The conventional limit of temperature is usually 37 ℃ ~ 63 ℃, and the suit...
Read More
Jun 17, 2025
view: 1233
45 cubic eo sterilizer FAQ 1.Internal volume not less than 40 m3 (please provide two options: standard dimension vs customized dimension to full utilise our available space). A: Yes, we provided two schemes, one is a 47m3 sterilizer and the other is...
Read More