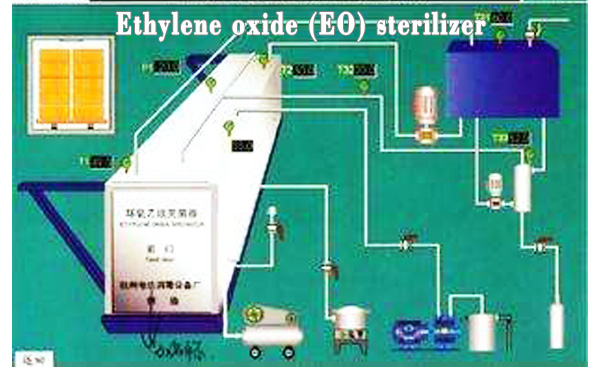
Definition Of EO
Ethylene oxide sterilization device is the key equipment of disposable sterile medical equipment manufacturing enterprises. There are special requirements for installation, operation and use management. Ethylene oxide is a broad-spectrum sterilization agent, which can kill all kinds of microorganisms, including spores, tuberculosis, bacteria, es, fungi, etc. at room temperature.
The basic ETO sterilization cycle consists of 5 stages (i.e., preconditioning and humidification, gas introduction, exposure, evacuation, and air washes) and takes approximately 8-10 hrs excluding aeration time.
In addition, Determine whether to use ethylene oxide sterilization based on product material and packaging type.
For products that are not compatible with high temperatures and ionizing radiation, ethylene oxide sterilization is a good choice. Due to its low-temperature process that can prevent material deformation or oxidation, ethylene oxide sterilization has a wide range of applications in medical device processing.
EO sterilization has the advantage of being compatible with multiple materials and is suitable for products that cannot withstand high temperature sterilization. Ethylene oxide can penetrate into the smallest gaps of the items to be disinfected. This feature allows it to be used on already packaged materials.
Products and packaging made from different materials require different sterilization processes.
EO Advantage
Convenient transportation and storage, no need of huge gas cylinder, no need of filtering gas, more importantly, CFC free. Environmental protection and safety.
1. It can kill all microorganisms, including bacterial spores.
2. Sterilized articles can be wrapped and packaged as a whole, which can keep sterile before use.
3 in contrast, EO does not corrode plastics, metals and rubber, and does not make items yellow or brittle.
4.It can penetrate irregular articles and sterilize them.
5.It can be used for sterilization of articles that cannot be soaked in disinfectant, sterilized by dry heat, pressure, steam and other chemical gases.
Matters needing attention
(1) Temperature and relative humidity have great influence on the sterilization effect of ethylene oxide, so the relevant conditions in the test should be strictly controlled.
(2) Ethylene oxide liquid can dissolve polyethylene, polyvinyl chloride, etc. do not drop its liquid on such articles. No matter liquid or gas, ethylene oxide can damage Celluloid products. Attention shall be paid during test.
(3) Ethylene oxide is inflammable and explosive. Fire and explosion proof measures shall be taken at the operation site, and there shall be no open fire operation and electric spark.
(4) Inhale too much ethylene oxide gas, can cause headache, vomiting and other toxic symptoms, serious cases can cause pulmonary edema and so on. Good ventilation shall be provided in the working environment. The concentration of ethylene oxide shall not exceed 1.82mg/m3 (1ppm) in 8h daily operation and 9.1mg/m3 (5.0ppm) in 15min operation. In case of poisoning symptoms, leave the site immediately. The lighter should breathe fresh air until the symptoms are eliminated; the heavier should be sent to the hospital in time for treatment.
Summary
At present, ethylene oxide is widely used to sterilize medical devices. . ethylene oxide is a flammable and explosive toxic gas, with the molecular formula of C2H4O, which has an aromatic ether smell. At 4 ° C, the relative density is 0.884, the boiling point is 10.8 ° C, and the density is 1.52g/cm3. At room temperature, it is easy to volatilize into a gas, which can cause explosion when the concentration is too high.
Jul 11, 2023
view: 1566
Inspection of mask after sterilization with EO Any product must pass the sterilization verification procedure to determine the sterilization process! Sterilization verification is generally carried out by half cycle method. This method is to determin...
Read More
Oct 30, 2020
view: 1929
Sterilization equipment shall comply with the applicable safety standards. Validation,IQ,OQ,PQ, The purpose of validation is to demonstrate that the sterilization process established in the process definition (see Clause 8) can be delivered effective...
Read More